5月8日,廣東藥監(jiān)局發(fā)布通告稱,根據(jù)企業(yè)申請,該局已于2024年3月依法注銷
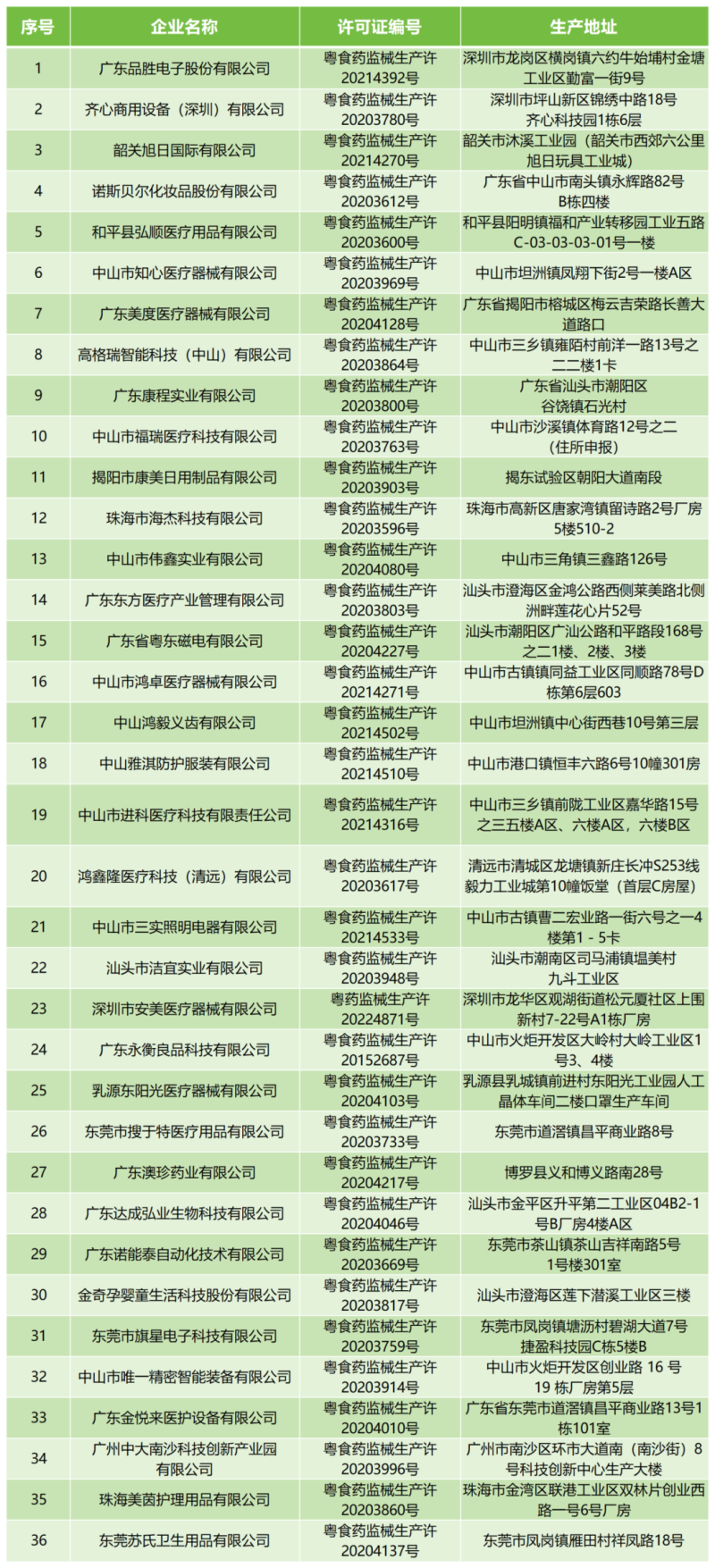
廣東品勝電子股份有限公司、齊心商用設(shè)備(深圳)有限公司、東莞市搜于特醫(yī)療用品有限公司等36家企業(yè)的《醫(yī)療器械生產(chǎn)許可證》,具體明細(xì)如下:
.
!" linktype="text" imgurl="" imgdata="null" data-itemshowtype="0" tab="innerlink" data-linktype="2" hasload="1" style="margin: 0px; padding: 0px; outline: 0px; text-decoration-line: none; -webkit-tap-highlight-color: rgba(0, 0, 0, 0); -webkit-user-drag: none; cursor: pointer; max-width: 100%; box-sizing: border-box !important; overflow-wrap: break-word !important;">
《重組膠原蛋白敷料》等6項行業(yè)標(biāo)準(zhǔn)征求意見中! !FDA對QSR 820進(jìn)行修訂,有哪些值得關(guān)注的點(diǎn)
?" linktype="text" imgurl="" imgdata="null" data-itemshowtype="0" tab="innerlink" data-linktype="2" hasload="1" style="margin: 0px; padding: 0px; outline: 0px; text-decoration-line: none; -webkit-tap-highlight-color: rgba(0, 0, 0, 0); -webkit-user-drag: none; cursor: pointer; max-width: 100%; box-sizing: border-box !important; overflow-wrap: break-word !important;">
?" linktype="text" imgurl="" imgdata="null" data-itemshowtype="0" tab="innerlink" data-linktype="2" hasload="1" style="margin: 0px; padding: 0px; outline: 0px; text-decoration-line: none; -webkit-tap-highlight-color: rgba(0, 0, 0, 0); -webkit-user-drag: none; cursor: pointer; max-width: 100%; box-sizing: border-box !important; overflow-wrap: break-word !important;">

